|
Enhancing childhood tuberculosis identification and treatment in the Philippines
PI: Anna Ma. Lena Lopez, Institute of Child Health and Human Development, University of the Philippines Manila--National Institutes of Health
NIH Partner: Karin Nielsen, David Geffen UCLA School of Medicine
Project dates: February 2015 - June 2019
 | Los Banos Health Care Center staff. (photo courtesy of PI Lopez)
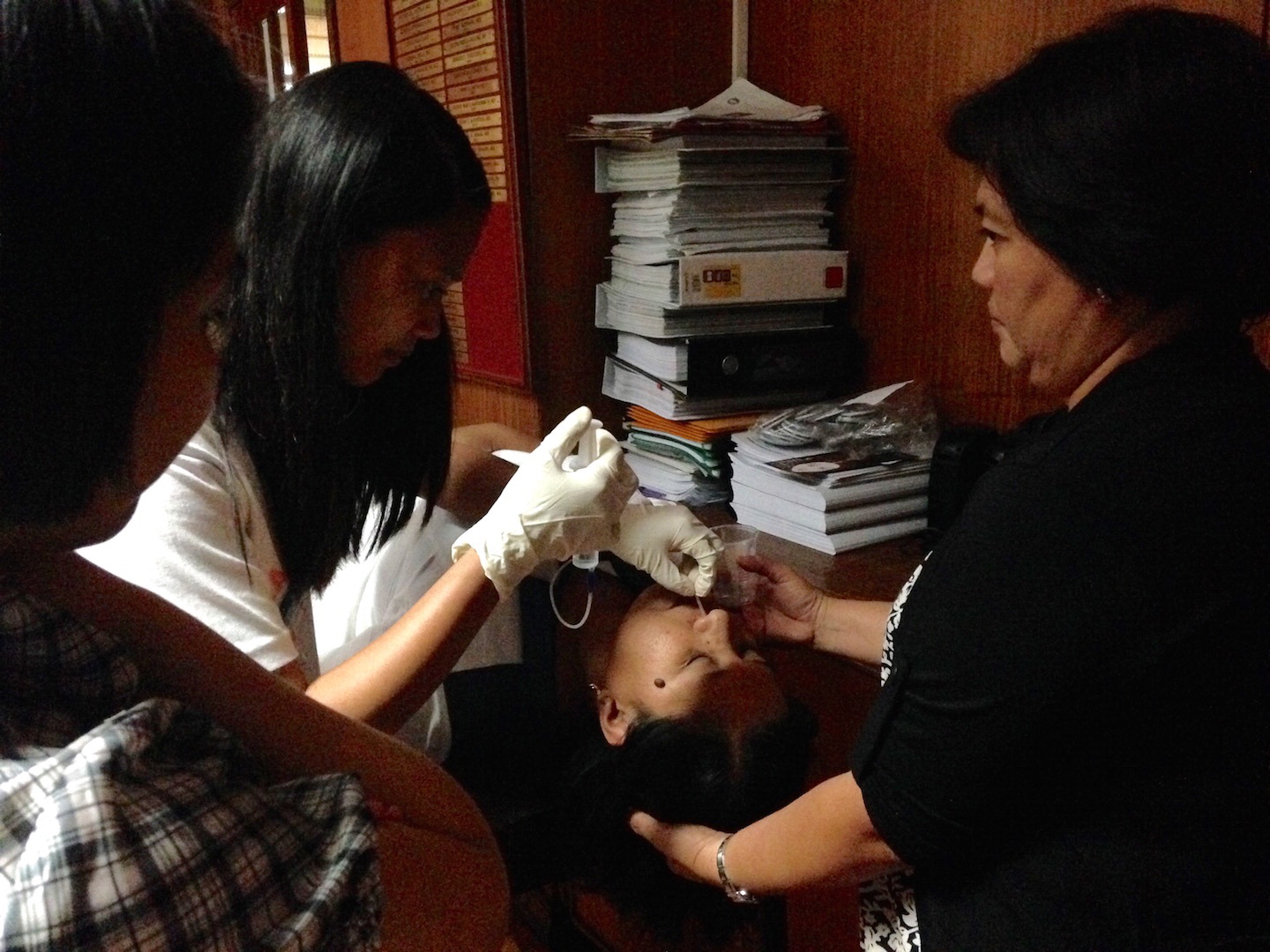
Training for diagnosis of tuberculosis using a nasopharyngeal aspirate (NPA). (photo courtesy of PI Lopez)
|
Project Overview
Childhood tuberculosis (TB) in the Philippines remains under-diagnosed, despite significant gains in the control of adult cases of TB. Learning from their experience in the previous year from a prospective community-based surveillance for TB in San Juan, Batangas, Philippines, this team designed various interventions to increase case detection and improve treatment outcomes of TB in the Philippines. They conducted a cluster-randomized field trial to assess the effect of an intervention package on tuberculosis case finding, treatment outcomes, and identification of children for isoniazid preventive therapy. The intervention package was implemented in randomly chosen barangays of two study sites. Aside from the childhood TB activities included in the National Tuberculosis and Control Programme (NTP) manual, no additional interventions would be offered in the rest of the barangays. This package included (1) enhanced contact tracing through the use of a mobile phone application (app) that automatically notifies barangay health workers (BHW) of patients enrolled in the NTP; (2) reinforcement of public health programs such as the Integrated Management of Childhood Illnesses (IMCI) and the nutrition program requiring referral of children with prolonged cough or fever, or with acute malnutrition for assessment of TB; (3) use of an USAID-funded field guide for health workers on childhood TB; (4) use of nasopharyngeal aspirates as a specimen for microbiologic testing among children who cannot expectorate; (5) use of a phone app that automatically informs patient’s follow-up date; and (6) use of text (or SMS) blasts to provide TB health education to enrolled study subjects.
Final Summary of Project Activities
The team conducted a cluster-randomized study in San Juan, Batangas, and Los Baños, Laguna, to assess the package of interventions surrounding TB control. San Juan and Los Baños have 42 and 19 barangays (communities), respectively, and each was randomly assigned as control or intervention for the trial. BHWs and midwives in the intervention group underwent training with regards to the study procedures, including the use of the mobile app for notification of positive tests of patients for faster contact tracing, nasopharyngeal aspiration, and referral of children assessed in the Integrated Management of Childhood Illness to the National TB Program (NTP) staff in the clinic. BHWs and midwives in the control group were informed of the study, but no additional training was provided. At the time of referral to the NTP, research staff obtained informed consent and assent (as appropriate) for study participation. Those in the intervention group who were unable to expectorate underwent nasopharyngeal aspiration for smear, Xpert, and culture.
From September 2015 to August 31, 2017, 1,017 children with presumptive TB and 1,075 adults with diagnosed TB were screened. Among the children screened, 586 (57.6%) and 431 (42.4%) were eventually enrolled in the intervention and control groups, respectively, and among adults enrolled 562 (52.2%) and 513 (47.7%) in the intervention and control groups, respectively. Among children, there were more presumptive TB cases in the intervention group, with more coming from Los Baños compared to San Juan among those who were enrolled. The majority of presumptive TB cases were identified during contact tracing followed by the IMCI program.
The team found that the package of interventions improved identification of presumptive TB cases but did not significantly increase detection of TB cases and latent TB infection cases. The majority of the presumptive TB cases were identified through contact tracing and the children were mostly exposed to TB cases in the household. The team's findings support that improving and consistently monitoring contact tracing will improve identification of presumptive TB cases. In addition, identifying cases in the IMCI also contributes to identification of presumptive TB cases. Nasopharyngeal aspiration was also tolerated and found acceptable by parents and children alike.
These are preliminary results from the study available at the time the final report was submitted in 2019. At that time, both San Juan and Los Baños were implementing screening for TB during IMCI visits, as well as consistently screening and inviting household contacts for TB. Dissemination of results was under way, as they will be helpful in further screening for TB and referring cases for treatment. Unfortunately, the PI Dr. Lopez passed away in February 2020 after a long illness.
Publication
A.L. Lopez, J.G. Aldaba, M. Moralez-Dizon, J.N. Sarol, J.V. Daag, M.C. Ama, P. Sylim, A. Salonga, and K. Saines-Nielsen. 2019. Urine Xpert MTB/RIF for the diagnosis of childhood tuberculosis. Int J Infect Dis 79: 44-46. https://doi.org/10.1016/j.ijid.2018.11.013
PEER Health Cycle 2 Recipients
|
|
|
|





